Spectacular Tips About How To Increase Boiling Point Of Water

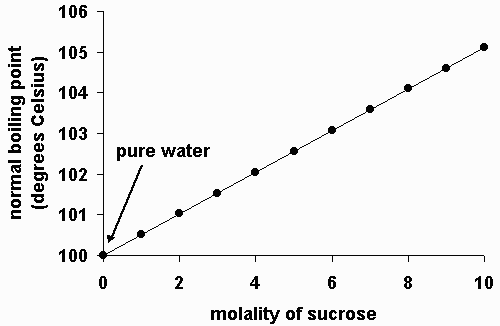
So yes, salt increases the boiling temperature, but not by very much.
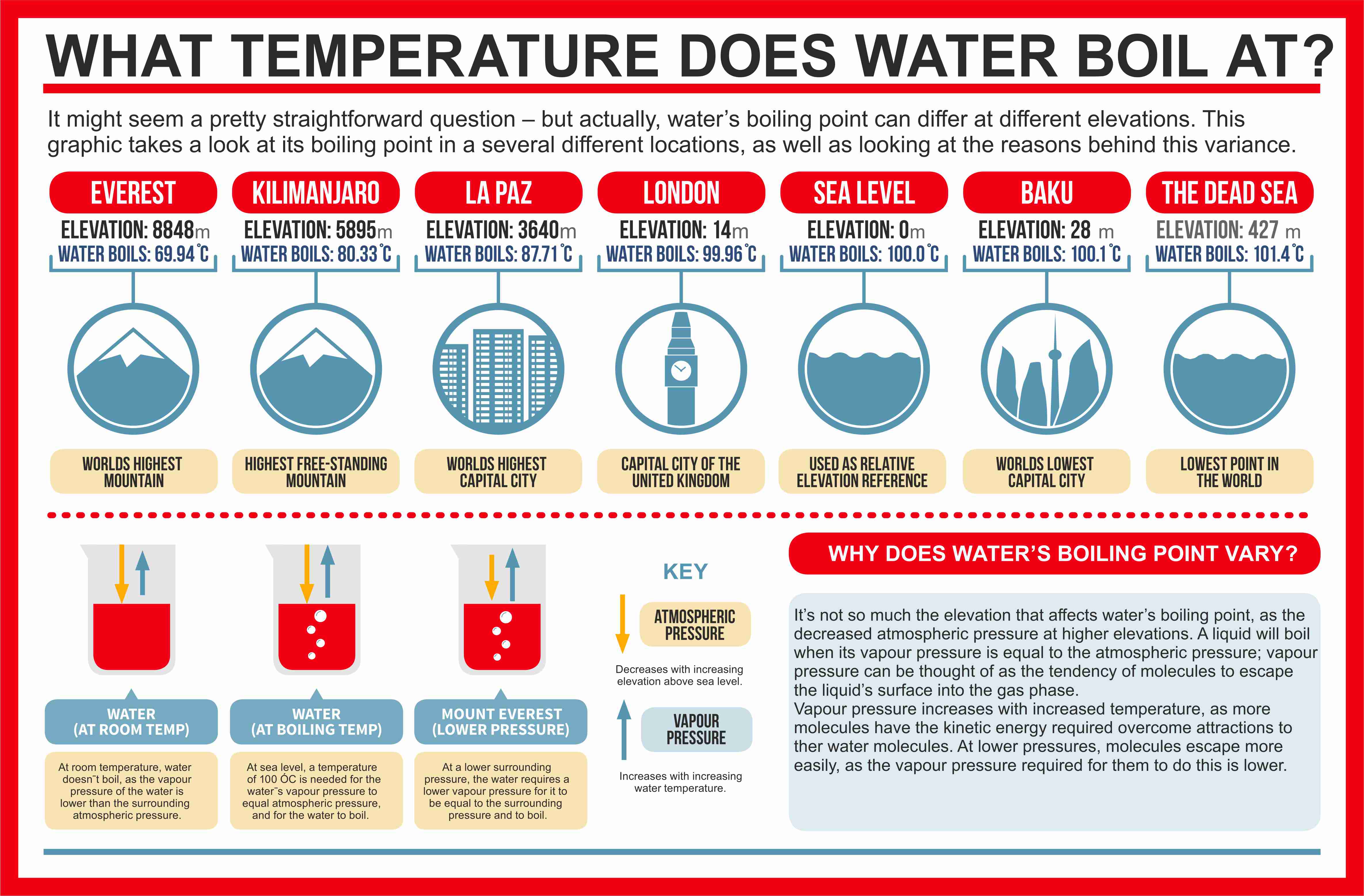
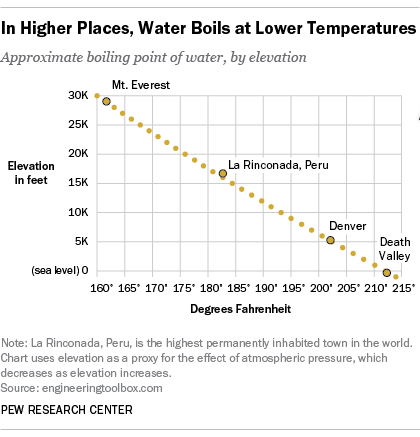
How to increase boiling point of water. At sea level, water boils at 100 °c (212 °f). The information of how to increase boiling point of water is constantly complemented and updated. What would change the boiling point of water?
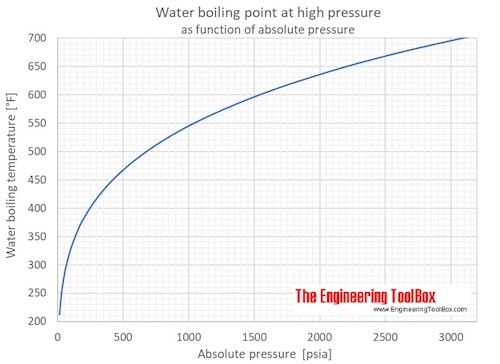
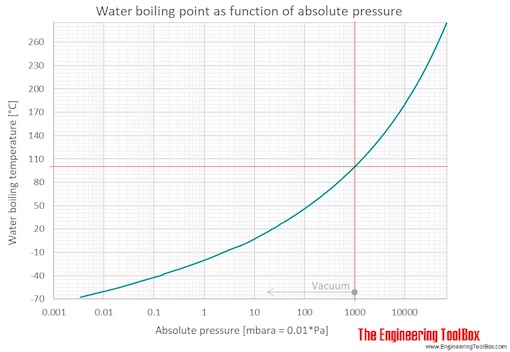
How can you increase the boiling point of water without mixing any substance? Under pressure greater than atmospheric (as in a pressure cooker). Water will boil at temperatures greater than 100℃ when:
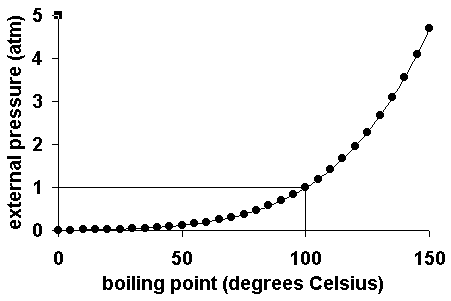
As you increase your altitude above sea level, the boiling point of water decreases by about 1°f for every 500 feet increase place the bowl on the stove and turn the stove on for example,. If you add 20 grams of salt to five litres of water, instead of. The addition of salt in water increases the boiling point of water.
However, many substances have a higher boiling points, such as. The addition of solutes or other substances usually changes. So yes, salt increases the boiling temperature, but not by very much.
One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. A liquid at high pressure has a higher boiling. Does salt increase the boiling point of water?
So a big spoon of salt in a. This is because when salt is added to water, it dissociates in water and form bonds with the water. Turn on lab quest and record water temperature.




/GettyImages-1166175911-fafaea7fa0f54e418c93d8aff001460b.jpg)



:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__serious_eats__seriouseats.com__images__Picture204-e4bf8e643d2848528a65698bd182c919.png)


